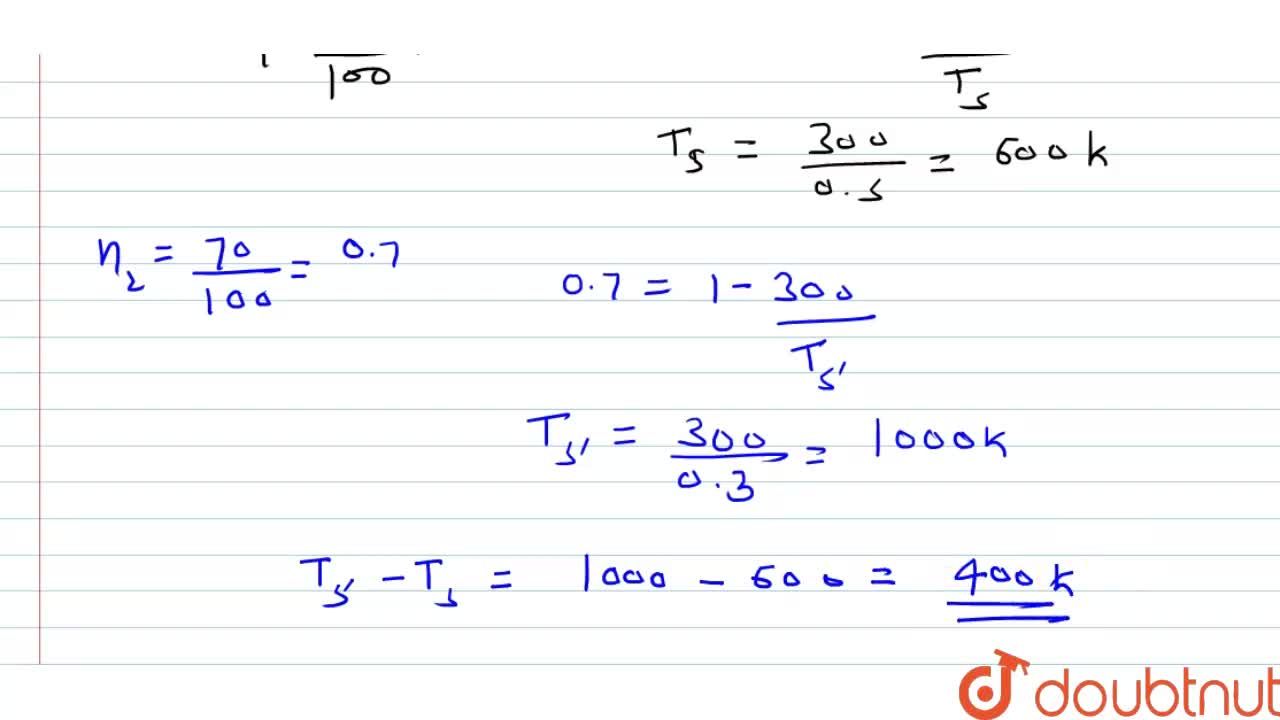
A first order reaction is 50% completed in 30 minutes at 300 K and in 10 minutes at 320 K, Calculate the activation energy of the reaction (R = 8.314 "JK mol"^(-1).)
21.rate of reaction increases four times when the temperature changes from 300 Kelvin to 320 Kelvin. Calculate the energy of activation of reaction assuming that it does not change with temperature

One mole of an ideal gas at 300 K in thermal contact with surroundings expands isothermally from 1.0 L to 2.0 L against a constant pressure of 3.0 atm . In this

SOLVED: Boltzmann constant: kB = 1.38 x 10-28 JK Thermal energy at 300 K: kBT = 0.0259 eV Energy unit conversion: 1eV = 1.60 x 10-19 J