
Draw and explain the Lewis structure for the azide ion, showing all its possible resonance structures. Give the name of the molecular geometry and the hybridization around the central atom. | Homework.Study.com

Draw resonance formulas for the azide ion, and for the nitronium ion. Decide which resonance formula is the best description of each ion. | Homework.Study.com

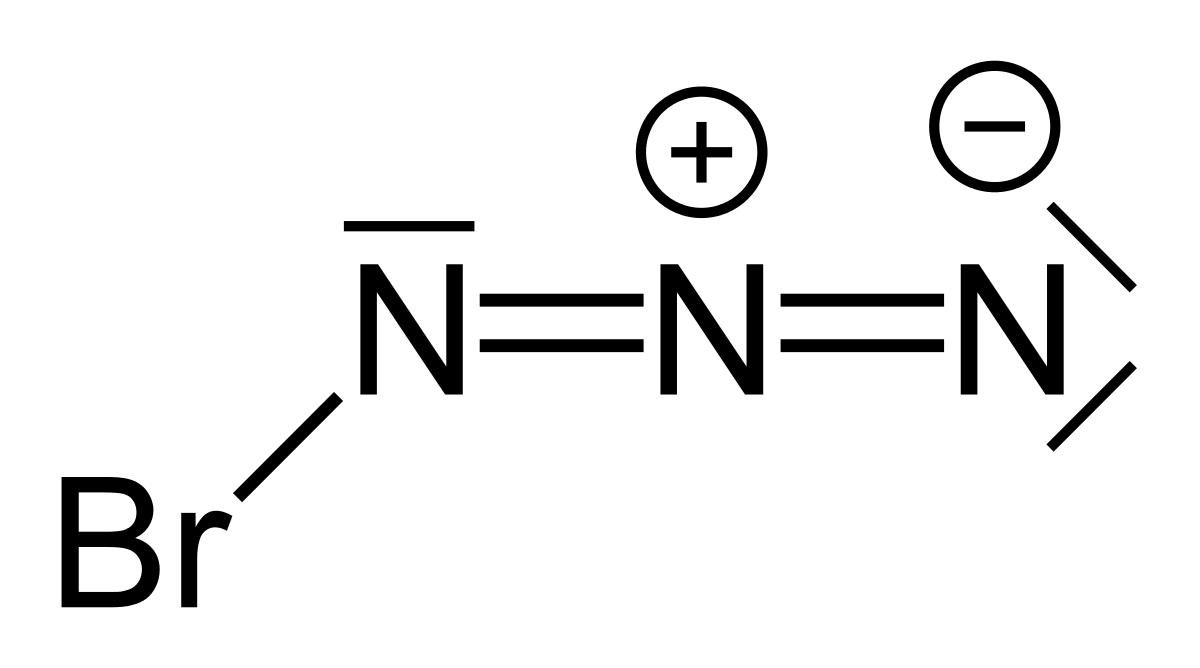

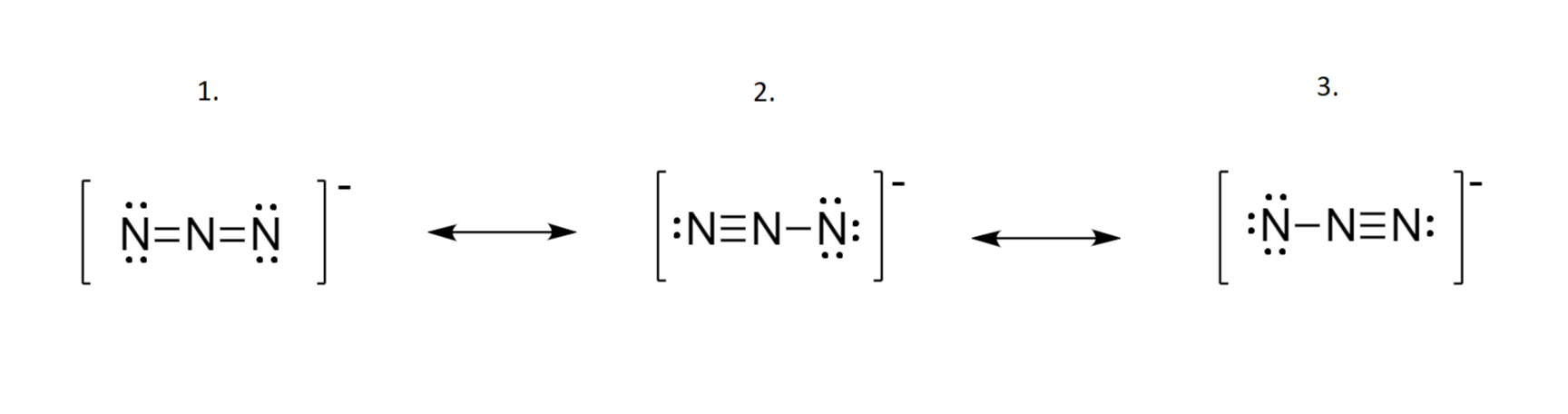


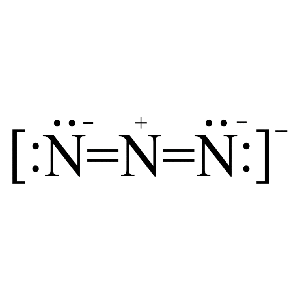




![Azides [N3(–)] - ChemistryScore Azides [N3(–)] - ChemistryScore](https://chemistryscore.com/wp-content/uploads/2019/11/Azides-N33-1024x206.png)

