Structures of (a) H2O, (b) Mg(H2O)62+, and (c) Fe(H2O)63+ and (d) the... | Download Scientific Diagram
Kc for CO(g) +H2O(g) ⇌ CO2(g) +H2(g) at 986°C is 0.63. A mixture of 1 mole H2O(g) - Sarthaks eConnect | Largest Online Education Community

Use the problem below to answer the question: 34 grams of carbon reacted with an unlimited amount of H20. - Brainly.com

Water , H2O, HOH molecule. It is inorganic hydroxy compound, oxygen hydride consisting of an oxygen atom and two hydrogen atoms. Sheet of paper in a c Stock Vector Image & Art - Alamy
![Calculate Kp for the reaction, C(s) + H2O(g) CO(g) + H2(g) at 990 K if the equilibrium concentration are as follows : [H2O] = 1.10 M, [CO] = [H2] = 0.2 M, Calculate Kp for the reaction, C(s) + H2O(g) CO(g) + H2(g) at 990 K if the equilibrium concentration are as follows : [H2O] = 1.10 M, [CO] = [H2] = 0.2 M,](https://dwes9vv9u0550.cloudfront.net/images/6653152/88b9752c-8929-4745-bc8f-8705cb2ad9b9.jpg)
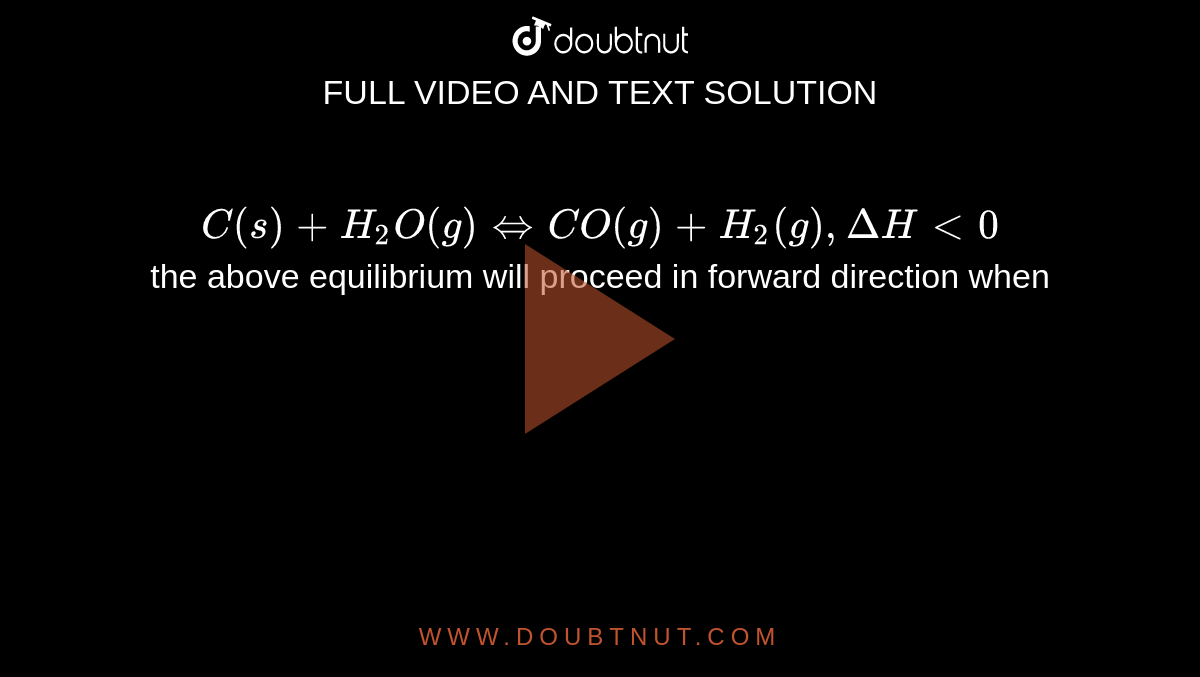
Calculate Kp for the reaction, C(s) + H2O(g) CO(g) + H2(g) at 990 K if the equilibrium concentration are as follows : [H2O] = 1.10 M, [CO] = [H2] = 0.2 M,

Question Video: Identifying the Chemical Formula of the Substance Produced When Carbon Dioxide Dissolves in Water | Nagwa