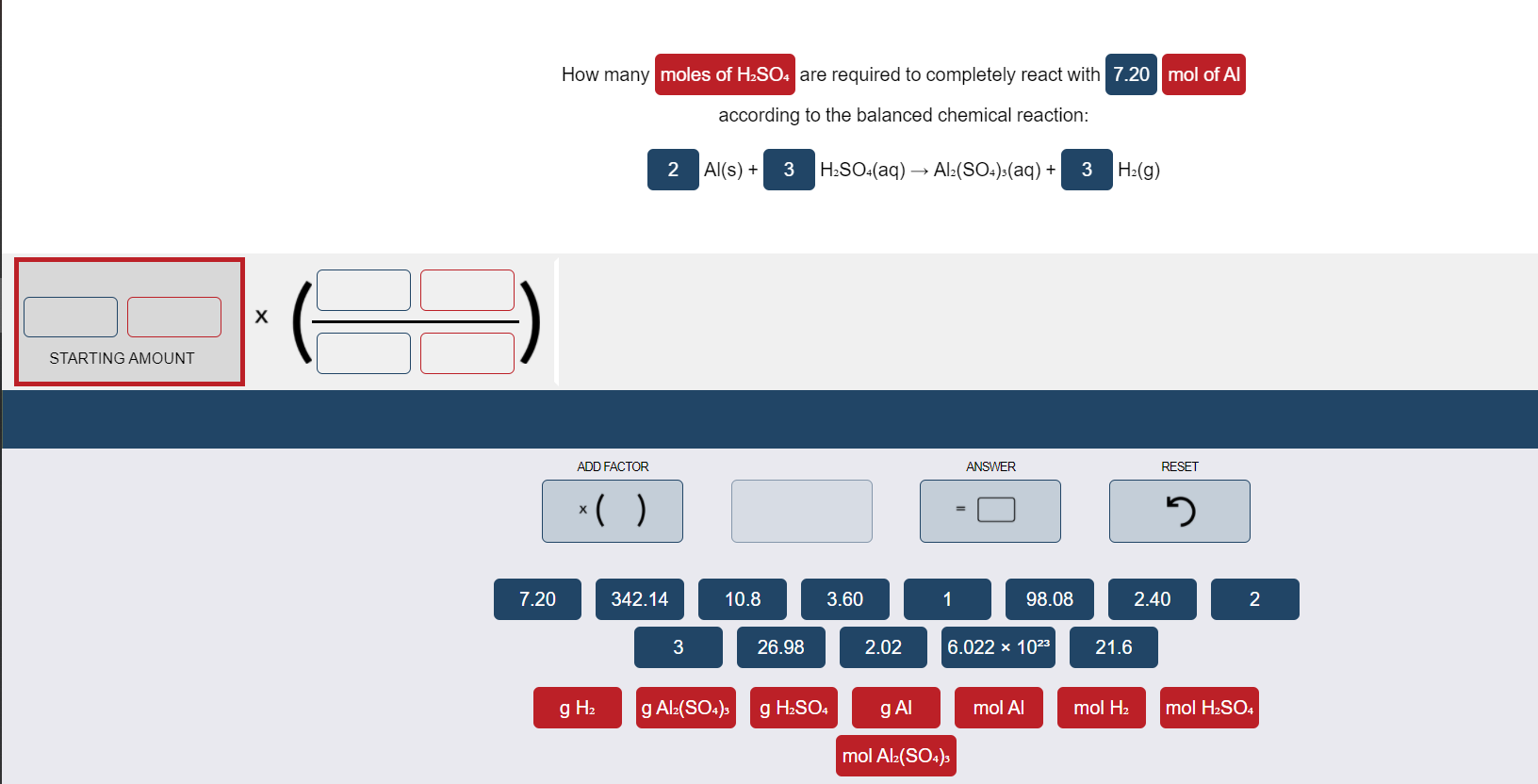
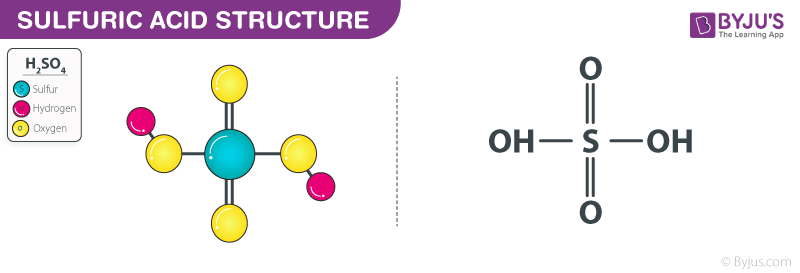
SOLVED: 4. What is the molar mass of 3.25 mol of H2SO4 Points) Enter your answer 5. What is the molar mass of 2.5 mol of (C3H5)2 ? Points) Enter your answer

The molecular mass of H2SO4 is 98 amu. Calculate the number of moles of each elements in 294 g of H2SO4

What volume of 0.250 mol/L sulfuric acid, H2SO4(aq) is needed to react completely with 37.2 mL of - Brainly.com

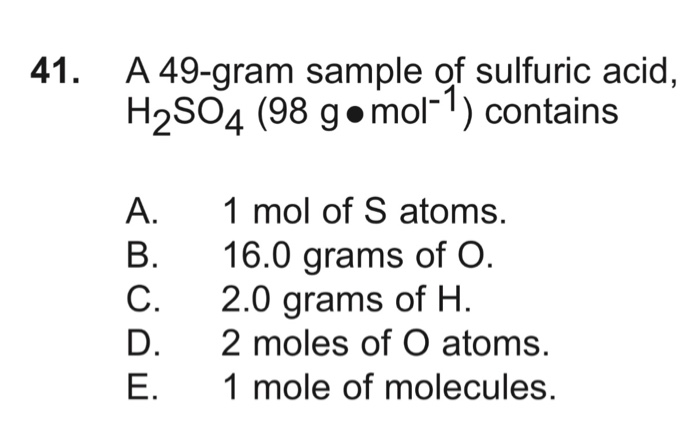
how many moles of h2so4 are present in 4 9 g h2so4 - Chemistry - Some Basic Concepts of Chemistry - 12591081 | Meritnation.com

Sulfuric Acid, Sulphuric Acid, Is A Highly Corrosive Strong Mineral Acid With The Molecular Formula H2SO4, Vector 3d Molecular Structure Royalty Free SVG, Cliparts, Vectors, And Stock Illustration. Image 78444709.
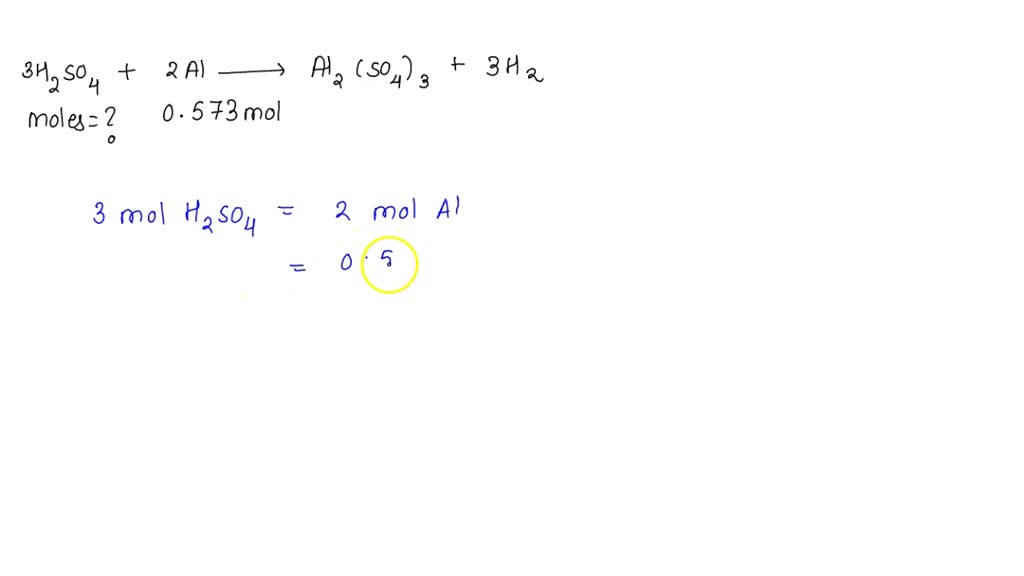
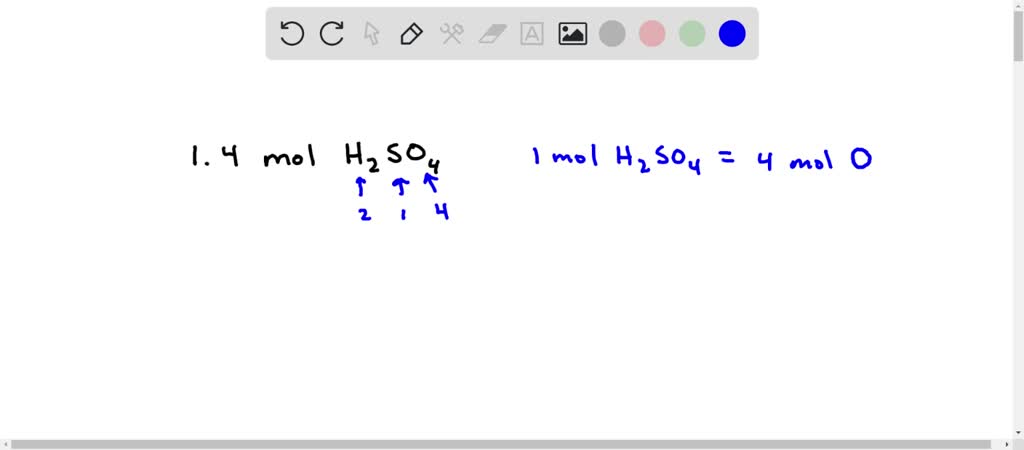

![Sulfuric Acid [H2SO4] Molecular Weight Calculation - Laboratory Notes Sulfuric Acid [H2SO4] Molecular Weight Calculation - Laboratory Notes](https://www.laboratorynotes.com/wp-content/uploads/2021/11/sulfuric-acid-molecular-weight-calculation.jpg)