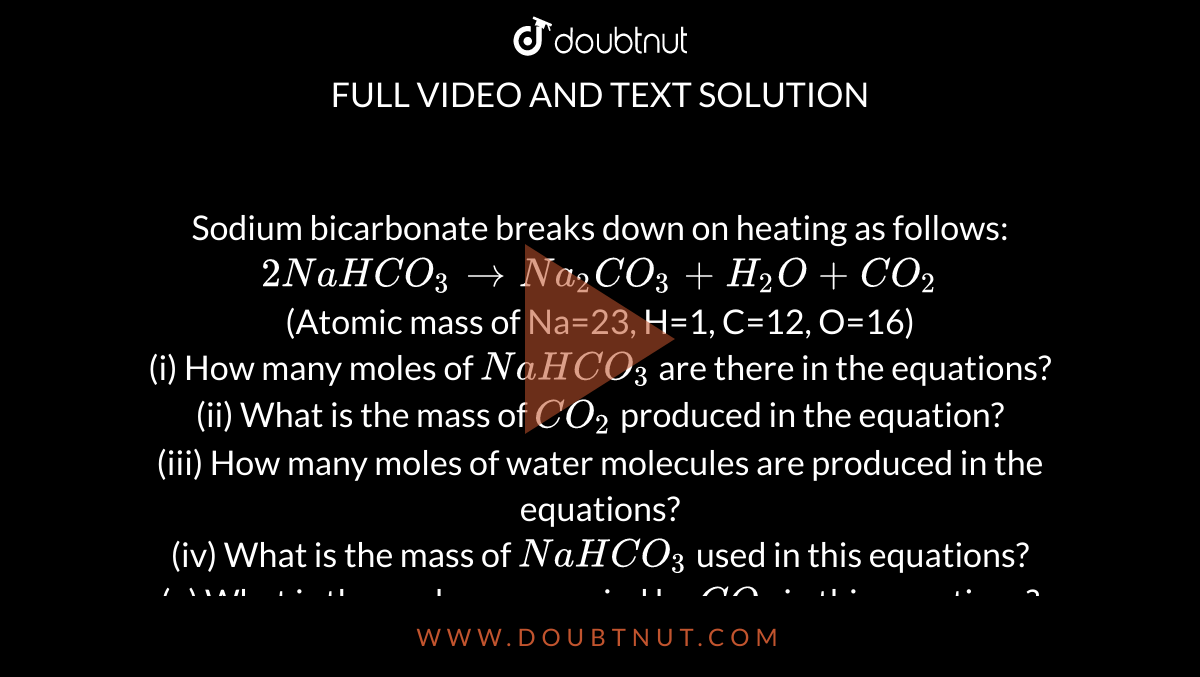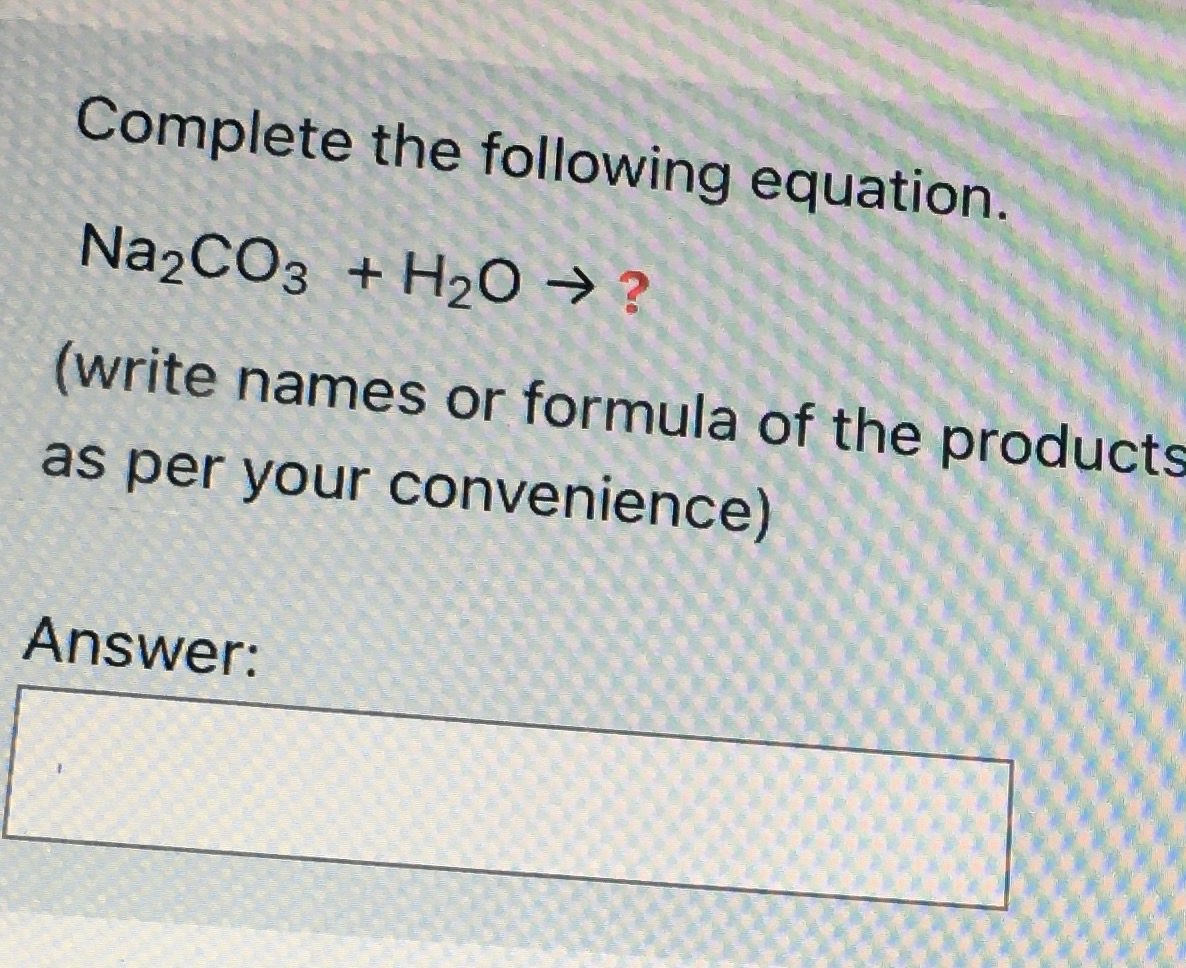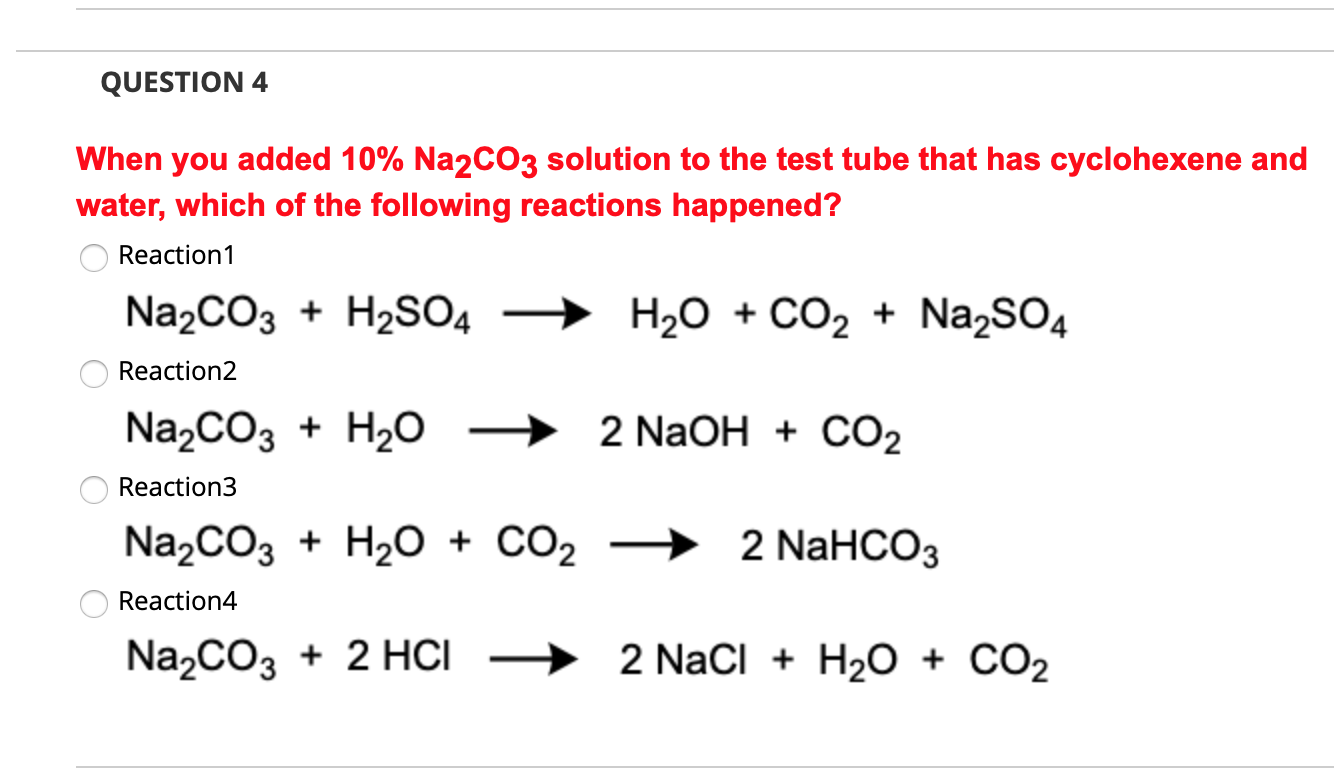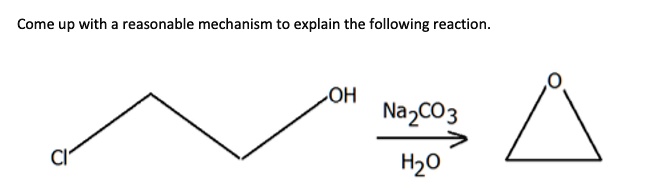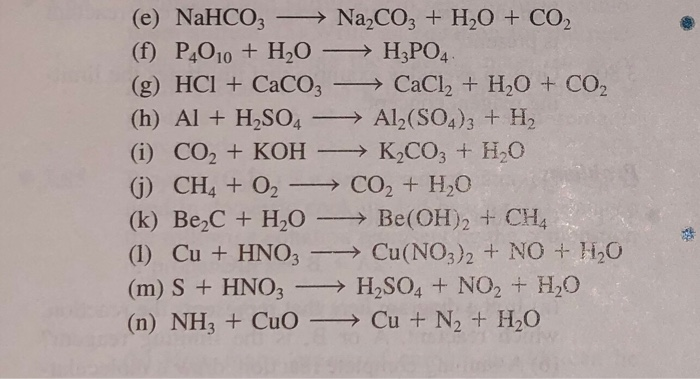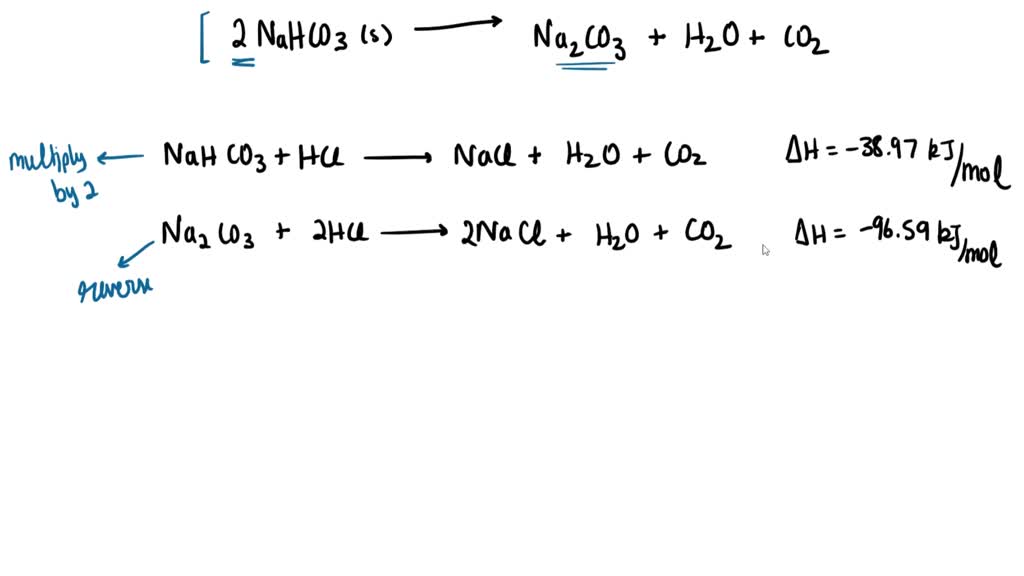
SOLVED: The following data are needed for this question. NaHCO3 (s) + HCl (aq) + NaCl (aq) + H20 (1) + CO2 (g) AH=-38.97 kJ mol-1 Na2CO3 (s) + 2HCl (aq) +

Chemical reaction-Rusty red iron(III) hydroxide precipitate (Fe(OH)3) in test tube formed reaction between iron(III) chloride (FeCl3) & sodium carbonate (Na2CO3): FeCl3+Na2CO3+H2O ->Fe(OH)3+NaCl+CO2 Stock Photo | Adobe Stock

5968-11-6, F.W. 124.00, Sodium Carbonate, Monohydrate, Granular, Reagent, ACS - 39G888|S1230-2.5KG76 - Grainger