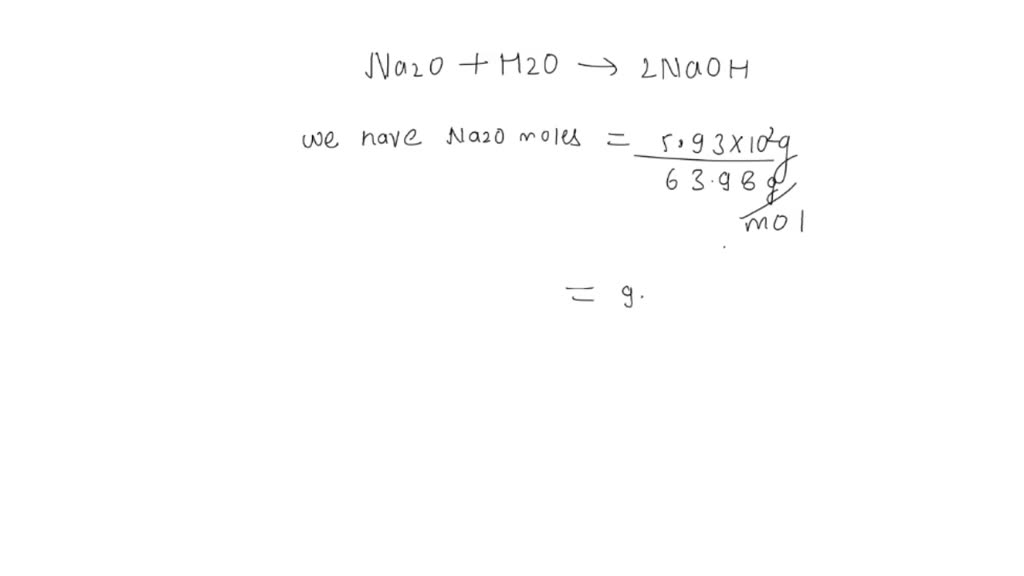3. Balance the following equations a CH4 +O2 = CO₂ + H₂Ob Na2O +H2O=NaOHc CA(OH)2+HCI=CaCI2+H2Od - Brainly.in

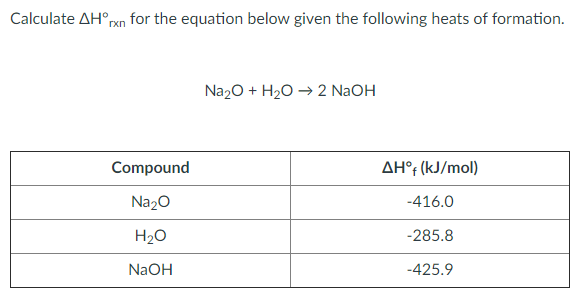
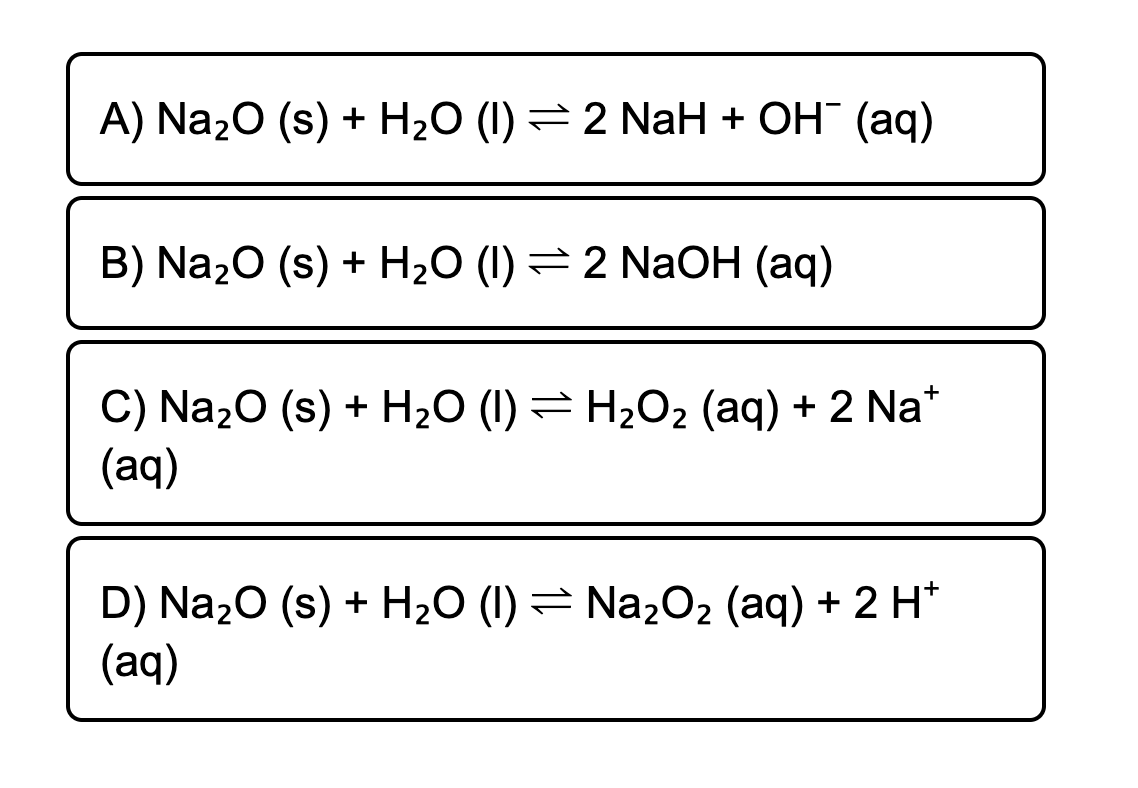
Given the following equation: Na2O + H2O ---> 2 NaOH 5. How many moles of NaOH are produced from 3.20 moles of Na2O? | Wyzant Ask An Expert

How to balance Na2O+H2O=NaOH|Chemical equation Na2O+H2O=NaOH|Na2O+H2O=NaOH balance equation - YouTube

Interzeolite conversion of zeolite MCM-49 into zeolite ZSM-35 in cyclohexylamine–hexamethyleneimine–Na2O–H2O containing systems - New Journal of Chemistry (RSC Publishing)

Internally consistent thermodynamic data for rock-forming minerals in the system SiO2-TiO2-Al2O3-Fe2O3-CaO-MgO-FeO-K2O-Na2O-H2O-CO2 - European Journal of Mineralogy Volume 9 Number 1 — Schweizerbart science publishers