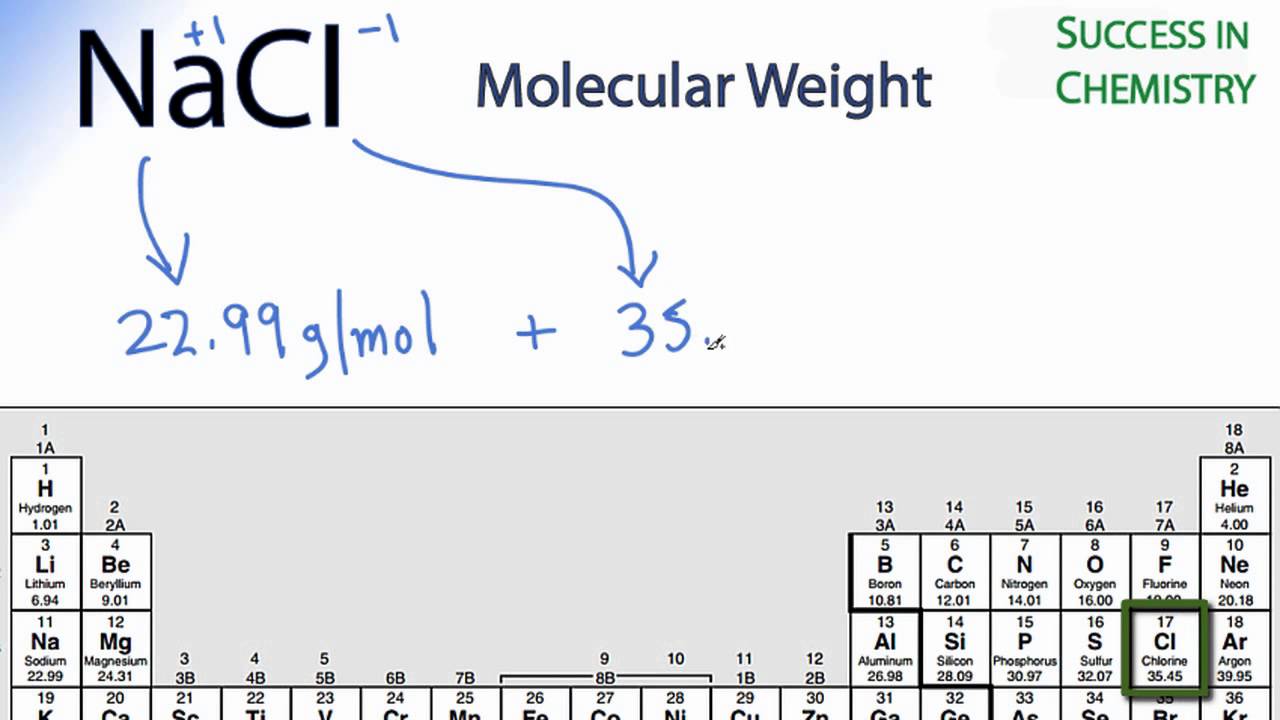
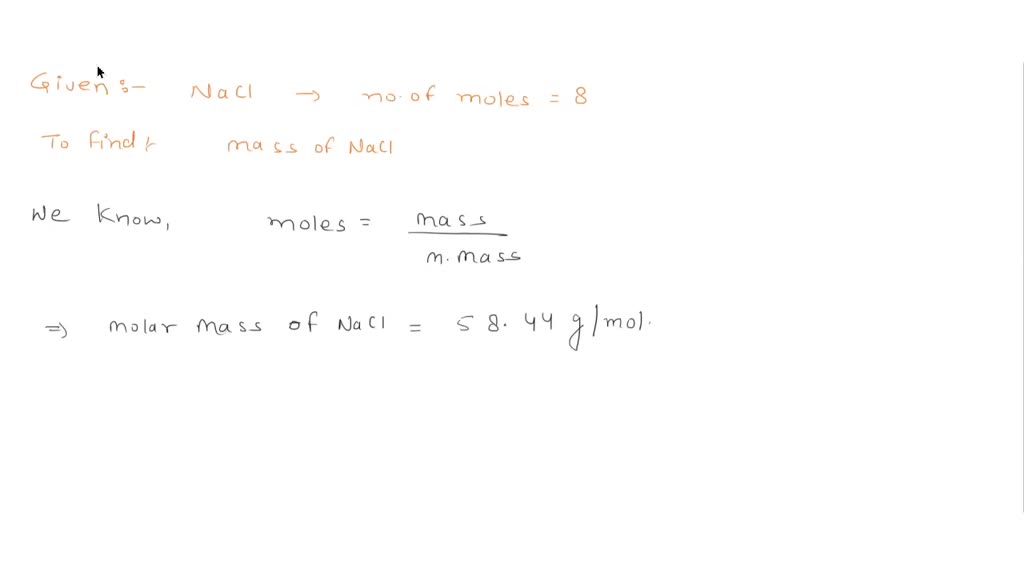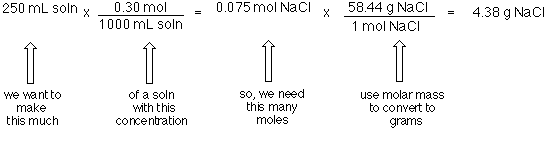Formula mass of NaCl is 58.45 g mol ^-1 and density of its pure form is 2.167 g cm ^-3 . The average distance between adjacent sodium and chloride ions in the

Variation of electrical conductivity of NaCl as a function of molar... | Download Scientific Diagram

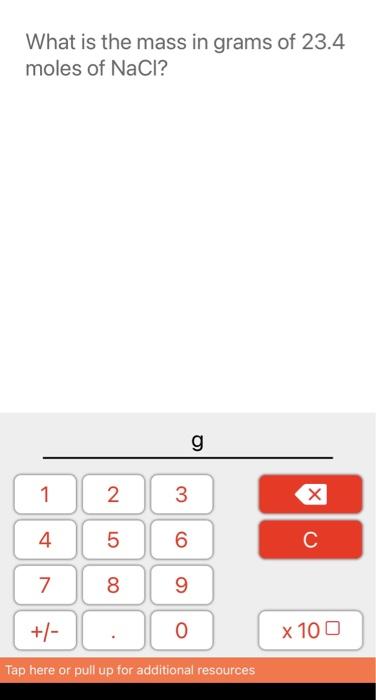
SOLVED: How many grams are in 3.40 mol of sodium chloride (NaCl)? Express your answer numerically in grams.

SOLVED: 7J Iinul Convert the mass of sodium chloride collected to moles. Moln Gci 66 ,44 3/mol 3089x Inul 0,00354(Mul 58 449 'Mul Divide both of your results from the preceding two

SOLVED: 1.a Calculate the moles of NaCl used to begin the reaction Hoicr mass cP Naci 6 8,44 9 mo 41.55 9 68,440 mol 0-71 moles mol Nacl GL mcl Calculate the

Formula mass of `NaCl` is `58.45g mol^(-1)` and density of its pure form is `2.167g cm^(-3)`. - YouTube